Individual Based Models In R
Individual-based models (IBMs; also called `agent-based models’) model systems of discrete individuals in silico (i.e., in a computer simulation, developed by writing and running code). The idea is to represent individuals (often, but not always, biological organisms) as discrete entities within within the code, and to simulate changes in individual composition and individual properties by modelling biological processes as algorithms within the code.
- Introduction: What is an individual-based model (IBM)?
- Getting started with IBMs in R
- Movement of individuals on a landscape
- Simulating individual movement over time
- Simulating individual birth
- Simulating individual death
- Predator-prey dynamics
- Putting it all together
- Conclusions
- Appendix with all code
1. Introduction: What is an individual-based model?
Biological models include a diverse set of tools that can be usefully applied to address a range of biological questions, from foundational principles of biological theory to more targeted questions about the dynamics of specific biological systems. In a forceful defence of theory and models in ecology, Caswell (1988) proposes the fundamental role of models in theory to be analogous to that of experiments in empirical biology. For more system-specific questions, complex modelling can be used with observational or experimental data to predict the dynamics of even highly complex systems (e.g., Aben et al. 2014). Whatever the question being asked, models help us to clarify the biological assumptions underlying our thinking, and to better understand the predictions that follow.
Unsurprisingly, the use of individual-based models has grown rapidly with the power and availability of computers (Grimm 1999; DeAngelis and Mooij 2005). Today, IBMs are widespread and well-established tools for all types of questions involving biological modelling. Two properties of IBMs stand out to make them especially advantageous. First, among-individual variation – a highly important property for understanding biological populations – is easy to model with an IBM. Whether it be variation among individuals in genotype, phenotype, or just spatial location, making individuals different in some relevant way is often a trivial matter of adding a bit more code. Second, because individuals are discrete, any biological processes being modelled are going to naturally include the kind of stochasticity that is inherent to all biological populations. There is often no need to explicitly model processes such as genetic drift, or demographic stochasticity underlying birth and death processes; these arise naturally from the structure of the model, and usually in a way that is biologically realistic. Hence, overall, IBMs are effective for addressing many (though, importantly, not all) questions relating to biological theory and predictive models.
My objective here is to get the reader started with writing their own IBMs. In particular, I hope to help the reader overcome one of the most challenging obstacles; where to start? Even for those who already have some programming experience, how to structure a model is not straightforward. In the text that follows, I will walk through the process of developing an IBM, step by step. By the end, we will have a fully working IBM of predator-prey dynamics. The code that follows is written, as much as possible, to be instructive and clear; it is not necessarily the most computationally efficient (i.e., fastest) way of coding a predator-prey IBM. It is written in R, which is not the best language for writing IBMs, but it is the language with which most biologists are probably familiar. Readers who get very interested in IBMs will eventually want to consider learning C or C++, which can improve computation speed by orders of magnitude. NetLogo is also a useful language to learn, as it was written specifically for individual-based modelling.
2. Getting started with IBMs in R
In an IBM, discrete individuals are represented by some sort of data structure in code (e.g., a list, table, array, or potentially some type of customised structure). There are several possible ways to do this, and the way that works best is probably most often a matter of the modeller’s preference and the coding language that they are using. In R, there are a few possibilities, but I usually find that the most intuitive way to model individuals is using a data table or array. For example, we can think about a two dimensional array as modelling a population of individuals, with rows representing discrete individuals and columns representing those individuals’ characteristics (‘characteristic’ is not a technical term here; I am loosely defining it to mean anything that relates to the individual in some way). The code below creates a two dimensional data array (inds) to model five individuals that have three characteristics each.
inds <- array(data = 0, dim = c(5, 3));
colnames(inds) <- c("characteristic_1", "characteristic_2", "characteristic_3");
rownames(inds) <- c("ind_1", "ind_2", "ind_3", "ind_4", "ind_5");
print(inds);
## characteristic_1 characteristic_2 characteristic_3
## ind_1 0 0 0
## ind_2 0 0 0
## ind_3 0 0 0
## ind_4 0 0 0
## ind_5 0 0 0
The row and column names are not required, but I have included them to make it easier to refer back to individuals and their characteristics. I have initialised all individual characteristics with a value of zero because it is still undecided what characteristics are actually being modelled. Characteristics can be anything we want them to be, from phenotypic traits, to alleles, to spatial locations, to some sort of temporary status (e.g., feeding or not feeding). The whole inds array is like a data set in which some number of measurements (columns) have been taken for some number of individuals (rows), except that we can make these measurements up to be whatever we want them to be, and manipulate them according to whatever rules we want to model. The objective is to carefully choose a set of characteristics and rules that model whatever biological process it is that we are trying to better understand.
We can start with some very simple individual characteristics. Assume that we want to model a population of animals moving around a landscape, and we want to include animal body mass and location as characteristics. We can use ‘characteristic_1’ in column 1 to randomly assign each individual a body mass according to some selected distribution, while ‘characteristic_2’ in column 2 and ‘characteristic_3’ in column 3 can be assigned random x and y locations, respectively. I will change the columns to reflect the new modelling decision for individual characteristics below.
colnames(inds) <- c("body_mass", "x_loc", "y_loc");
Let us say that body mass is normally distributed around a value of 23 with a standard deviation of 3. If it helps the reader to have a concrete example in mind to visualise, this is roughly the body mass distribution in kilograms of female roe deer (Pettorelli et al. 2002). Nevertheless, it is usually not important to have any particular species in mind unless the model is being constructed to address a very targeted question. We can give each individual a body mass randomly sampled from 𝒩(23, 3) with the code below.
inds[, 1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
Note that dim(inds) returns the dimensions of the array inds where element 1 (i.e., dim(inds)[1]) is the number of rows (5 in this case) and element 2 is the number of columns (3 in this case). Also note that that in the code above, inds[, 1] includes all of the values in column 1; it is the same as if I had typed inds[1:5, 1]. Each individual in the array is now modelled with a unique body mass in the first column.
## body_mass x_loc y_loc
## ind_1 27.45442 0 0
## ind_2 24.24773 0 0
## ind_3 20.96542 0 0
## ind_4 23.68664 0 0
## ind_5 21.95709 0 0
We can now give each individual its own location. Assume that these individuals all occupy some location on an 8 × 8 grid like the one shown below.
We do not actually need to code this grid to model individual locations or movement, but it might help to think of each individual in our model as occupying some square (i.e., ‘cell’) on the grid above. This is just one way to model individual position on a spatially explicit landscape, but it is often especially useful because it allows us to use natural numbers to define how far away individuals are from one another (number of cells away), and when individuals occupy the exact same location. We can now set individual x and y locations into columns 2 and 3, respectively, by sampling from a vector of numbers 1 to 8 with replacement (sampling with replacement ensures that more than one individual can occupy the same x or y location).
inds[, 2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[, 3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
Each individual now occupies a location on the landscape, as shown now in columns 2 and 3.
## body_mass x_loc y_loc
## ind_1 27.45442 7 2
## ind_2 24.24773 5 5
## ind_3 20.96542 2 7
## ind_4 23.68664 5 2
## ind_5 21.95709 2 7
We can even plot the individuals’ x and y locations to see how they are spatially distributed.
plot(x = inds[,2], y = inds[,3], pch = 20, cex = 4, xlim = c(1, 8),
ylim = c(1, 8), xlab = "x location", mar = c(5, 5, 1, 1),
ylab = "y location", cex.lab = 1.5, cex.axis = 1.5);
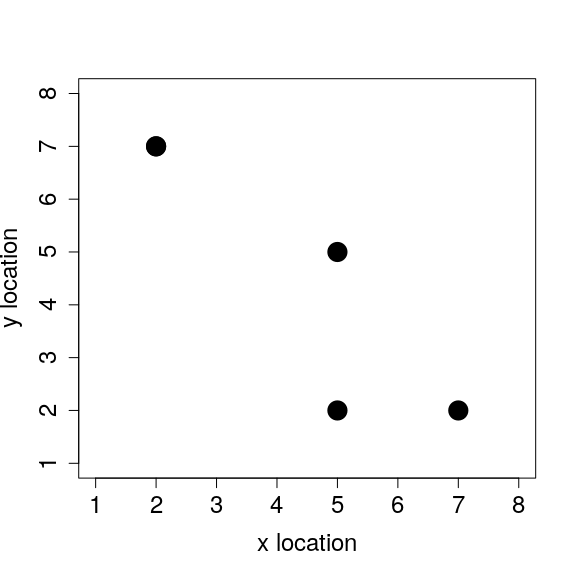
This is really all that is needed to get started. It is, of course, possible to add any number of individuals (rows) or individual characteristics (columns). And it is not necessary to model body mass or even spatial location if these are not useful characteristics for modelling the system of interest. What matters is that we have a discrete number of individuals with some set of characteristics that are relevant for whatever question it is that we want to answer. These individuals and characteristics will change over the course of simulating an IBM according to whatever rules we specify. This can include rules for movement, birth, death, or anything else that we might think is important to model in the system. In the next section, I consider some simple rules for individual movement.
3. Movement of individuals on a landscape
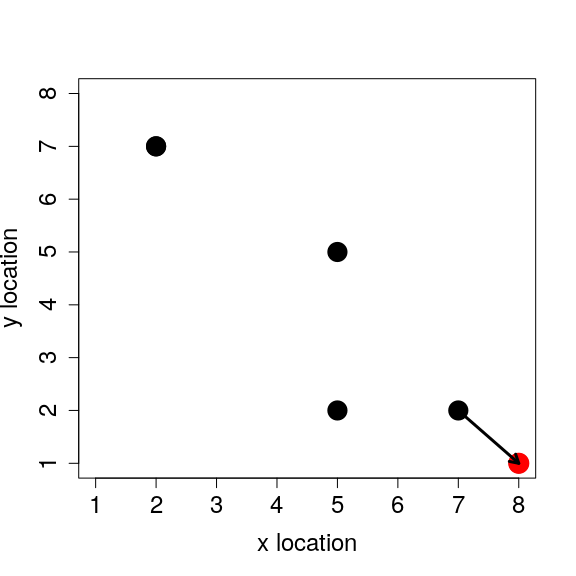
Each of the individuals that we initialised in the previous section has an x and y location on the landscape. To model individual movement, we need to come up with some rule for how these locations change. The simplest model of movement is to allow individuals to increase or decrease their x and y locations by 1 cell, so ind_1 with an x location of 7 might move along the x axis to a new x location of 8 or 6. The individual might likewise move up or down one cell on the y axis. To model random movement to any of the eight cells surrounding a focal individual’s current location (thereby also allowing diagonal movement), we can sample two random integer values from the set { − 1, 0, 1} with replacement and equal probability. One value is for the x location, and the other is for the y location. Movement is modelled by adding each value to the current x and y locations; the code below moves ind_1 in the first row of inds.
x_move <- sample(x = c(-1, 0, 1), size = 1);
y_move <- sample(x = c(-1, 0, 1), size = 1);
inds[1, 2] <- inds[1, 2] + x_move;
inds[1, 3] <- inds[1, 3] + y_move;
We can see the new location in the table inds, and we can plot the individuals’ locations again with the location of ind_1 shown in red. The arrow below indicates the original cell from which ind_1 moved.
## body_mass x_loc y_loc
## ind_1 27.45442 8 1
## ind_2 24.24773 5 5
## ind_3 20.96542 2 7
## ind_4 23.68664 5 2
## ind_5 21.95709 2 7
This was only one individual, but we can move all of the individuals simultaneously according to our movement rule if we sample a vector of x_move and y_move that matches the number of individuals in the array. The code below samples two movement vectors that are the same length as the number of rows in inds (i.e., one value of -1, 0, or 1 sampled for each individual’s x and y location).
x_move <- sample(x = c(-1, 0, 1), size = dim(inds)[1], replace = TRUE);
y_move <- sample(x = c(-1, 0, 1), size = dim(inds)[1], replace = TRUE);
inds[, 2] <- inds[, 2] + x_move;
inds[, 3] <- inds[, 3] + y_move;
We can now see their new positions in the data array columns 2 and 3, and compare the new values in these columns with the old one above to verify that individuals have indeed moved, at most, one cell in any direction.
## body_mass x_loc y_loc
## ind_1 27.45442 7 2
## ind_2 24.24773 4 4
## ind_3 20.96542 2 6
## ind_4 23.68664 6 2
## ind_5 21.95709 2 8
We can see that the x and y locations have not changed by more than a single value. Double-checking code in this way is critical at every step of the modelling process. It is very easy to overlook a coding error that might cause a much different rule than the one intended. To be especially vigilant, it is often a good idea to give each unique process in an IBM its own function. There are at least three benefits of coding this way. First, it will later allow us to test each process in the IBM independently, so that if one part of the model does not appear to be working as it should, then we can narrow down the problem more easily by checking each function. Second, it makes it easier to change the order of operations in our IBM (e.g., whether movement happens before, or after, birth or death processes). Third, it makes the code easier to read; rather than having to scan through all of the code at once to understand a model, we can break things down piece by piece (this will become clearer later). Below, I write a function to move individuals on the landscape according to the movement rules described above. Note that the code is essentially the same as in the block above, but structured slightly differently to make it into a more readable function.
movement <- function(inds, xloc = 2, yloc = 3){
total_inds <- dim(inds)[1]; # Get the number of individuals in inds
move_dists <- c(-1, 0, 1); # Define the possible distances to move
x_move <- sample(x = move_dists, size = total_inds, replace = TRUE);
y_move <- sample(x = move_dists, size = total_inds, replace = TRUE);
inds[, xloc] <- inds[, xloc] + x_move;
inds[, yloc] <- inds[, yloc] + y_move;
return(inds);
}
We now have a function movement, which takes in three arguments (inds, xloc, and yloc) and returns an updated array of individuals with new x and y locations. The argument inds is the array of individuals that we want to move, while xloc and yloc are the column numbers where the x and y locations of individuals are stored, respectively (default values are set to 2 and 3). Of course, we could have not included xloc and yloc as function arguments, and instead just added 2s and 3s where xloc and yloc appear within the movement function. But specifying these arguments makes the function more flexible. Now if we want to change which of the individual array inds columns specify location, for whatever reason, we need only specify a different xloc and yloc when calling the movement function. We could do the same with move_dists, if we wanted to make movement even more flexible.
To move individuals in the array inds, we now only need to call the movement function. As a reminder, here is what inds looks like now.
## body_mass x_loc y_loc
## ind_1 27.45442 7 2
## ind_2 24.24773 4 4
## ind_3 20.96542 2 6
## ind_4 23.68664 6 2
## ind_5 21.95709 2 8
Below moves the individuals with only one line of code using our new function. Note that we do not need to specify xloc and yloc arguments because we are happy with the default values of 2 and 3, respectively.
inds <- movement(inds);
The new inds array is below.
## body_mass x_loc y_loc
## ind_1 27.45442 7 2
## ind_2 24.24773 3 3
## ind_3 20.96542 2 7
## ind_4 23.68664 5 2
## ind_5 21.95709 1 7
Now that we have some code that has been tested and works as intended, we can use it to simulate the movement of individuals over multiple time steps. I will show how to do this in the next section.
4. Simulating individual movement over time
To simulate the movement of our individuals over time, we now need to call the movement function that we wrote in the previous section multiple times. We can define the total number of time steps to simulate over below.
time_steps <- 20;
To run a simulation of individuals moving for twenty time steps, we now need to use a loop. A while loop in which some variable ts (indicating ‘time step’) increases from 0 to 19 is probably the easiest way to code the simulation.
ts <- 0;
while(ts < time_steps){
inds <- movement(inds);
ts <- ts + 1;
}
The code above first sets the starting time step to a value of 0. Within the while loop, individuals in the inds array are moved, then the time step value is increased by one. The loop continues calling movement to move individuals until the condition in the while loop (ts < time_steps) is satisfied. After the loop is finished, individuals have moved twenty times from their initial starting locations. Here is where they are located now.
## body_mass x_loc y_loc
## ind_1 27.45442 0 -3
## ind_2 24.24773 9 -3
## ind_3 20.96542 3 14
## ind_4 23.68664 4 -2
## ind_5 21.95709 1 7
The code appears to have worked, but there is a problem that we have not yet considered. We originally defined our landscape as an 8 × 8 grid, but there is nothing in the movement rules preventing individuals from moving off of the landscape. This might not matter to us; perhaps we are happy to assume that some individuals will migrate outside of the area of interest. But often we want the population to be enclosed, and for individuals to not leave it by moving outside the bounds that we have set. If this is the case, then we need to decide what happens when individuals move past the edge of the landscape. There are several possibilities for what to do when individuals move outside of the landscape boundary.
- Place them back onto the boundary edge (i.e., a sticky landscape edge).
- Change their direction at the boundary edge (i.e., a reflecting landsacpe edge).
- Have them move to the opposite side of the landscape (i.e., a torus landscape with no edge)
In my experience, 3 is the most popular option, perhaps because it eliminates the problem of what to do with individuals that leave the edge of the landscape altogether by making it so that no edge exists. For now, I will demonstrate a reflecting edge instead because it is slightly easier to code. To make individuals that move one cell past the boundary of the landscape instead move back one cell toward the centre of the landscape, we can add some new code to the movement function.
movement <- function(inds, xloc = 2, yloc = 3, xmax = 8, ymax = 8){
total_inds <- dim(inds)[1]; # Get the number of individuals in inds
move_dists <- c(-1, 0, 1); # Define the possible distances to move
x_move <- sample(x = move_dists, size = total_inds, replace = TRUE);
y_move <- sample(x = move_dists, size = total_inds, replace = TRUE);
inds[, xloc] <- inds[, xloc] + x_move;
inds[, yloc] <- inds[, yloc] + y_move;
# ========= The reflecting boundary is added below
for(i in 1:total_inds){ # For each individual i in the array
if(inds[i, xloc] > xmax){ # If it moved passed the maximum xloc
inds[i, xloc] <- xmax - 1; # Then move it back toward the centre
}
if(inds[i, xloc] < 1){ # If it moved below 1 on xloc
inds[i, xloc] <- 2; # Move it toward the centre (2)
}
if(inds[i, yloc] > ymax){ # If it moved passed the maximum yloc
inds[i, yloc] <- ymax - 1; # Then move it back toward the centre
}
if(inds[i, yloc] < 1){ # If it moved below 1 on yloc
inds[i, yloc] <- 2; # Then move it toward the centre (2)
}
}
# ========= Now all individuals should stay on the landscape
return(inds);
}
With the new movement function, let us first initialise a new population of inds in the same way as before.
inds <- array(data = 0, dim = c(5, 3));
colnames(inds) <- c("body_mass", "x_loc", "y_loc");
rownames(inds) <- c("ind_1", "ind_2", "ind_3", "ind_4", "ind_5");
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[,2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[,3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
Here is what the newly initialised individuals in inds look like.
## body_mass x_loc y_loc
## ind_1 26.47984 5 3
## ind_2 20.69655 7 7
## ind_3 15.76510 6 4
## ind_4 21.52540 8 1
## ind_5 25.15732 1 2
With our new movement function defined above, we can again simulate movement over twenty time steps. This time, though, we should not see any individuals leaving the 8 × 8 grid.
ts <- 0;
time_steps <- 20;
while(ts < time_steps){
inds <- movement(inds);
ts <- ts + 1;
}
print(inds);
## body_mass x_loc y_loc
## ind_1 26.47984 4 1
## ind_2 20.69655 8 4
## ind_3 15.76510 7 3
## ind_4 21.52540 6 2
## ind_5 25.15732 5 2
Individuals have now moved from their original locations after 20 time steps, but none of the individuals has an x or y location less than 1 or greater than 8, meaning that they are all still on the landscape. Hence, we now have a working IBM in which individuals move around randomly on an 8 × 8 grid. We have initialised five individuals with three characteristics, but as long as we have x and y locations stored in inds columns, we could always increase the number of individuals and characteristics in the model and add new rules (functions) within the above while loop. I will show how to do this in the next section, but before moving on, I want to first show some ways to keep track of what is already going on in our IBM.
Note that in using the loop immediately above, we have successfully modelled animal movement over 20 time steps, but we have not retained any information about where each individual was located within these 20 time steps. We only know the x and y locations where the individuals started at time_step == 0, and where each of them ended at time_step == 19. This might not be a problem; perhaps the intervening locations do not really matter to us. Or maybe this would be too much information to store [1]. But suppose that we actually wanted to record the locations of all of these individuals in each time step, and knew that storing these data would not be an issue. Doing so would allow us to reconstruct the movement patterns of each individual and see how the whole population moves from time step 0 to time step 20. In R, we can do this easily by creating a new list and storing the inds array as a list element in each time step.
ts <- 0;
time_steps <- 20;
inds_hist <- NULL; # Here's the list
while(ts < time_steps){
inds <- movement(inds);
ts <- ts + 1;
inds_hist[[ts]] <- inds; # Add to list
}
print(inds);
## body_mass x_loc y_loc
## ind_1 26.47984 1 6
## ind_2 20.69655 5 6
## ind_3 15.76510 1 7
## ind_4 21.52540 6 4
## ind_5 25.15732 1 3
With the addition of the inds_hist list, we can see where every individual was in each time step. For example, we can look at individual movement in the first three time steps by printing inds_hist elements below (note that inds_hist[[1:3]] does not work in R – we need to print line by line, or use a loop).
print(inds_hist[[1]]);
## body_mass x_loc y_loc
## ind_1 26.47984 4 2
## ind_2 20.69655 7 3
## ind_3 15.76510 7 2
## ind_4 21.52540 6 2
## ind_5 25.15732 6 2
print(inds_hist[[2]]);
## body_mass x_loc y_loc
## ind_1 26.47984 4 1
## ind_2 20.69655 6 3
## ind_3 15.76510 6 3
## ind_4 21.52540 6 1
## ind_5 25.15732 5 3
print(inds_hist[[3]]);
## body_mass x_loc y_loc
## ind_1 26.47984 5 1
## ind_2 20.69655 6 2
## ind_3 15.76510 6 3
## ind_4 21.52540 5 1
## ind_5 25.15732 4 2
The inds_hist list essentially stores the entire history of the individuals moving over the course of the simulation. Storing this kind of information is very useful for reconstructing the history of a simulation to understand what is going on. As more biological processes are added (e.g., birth, death, predation, etc.), we can effectively take a perfect snapshot of each point in time in the system that we are modelling. Often the sheer size of an IBM prohibits a complete record of every individual’s history, but as the IBM grows, we can choose what information is important to retain and analyse. For now, because the the model is small (few individuals and time steps), we can simply keep everything. Model analysis then becomes a process of extracting the relevant information that we have stored during the simulation and using it to make meaningful biological inferences. We could, for example, see where individual 1 has been over the 20 time steps by extracting the information from inds_hist and storing it in a new table.
ind1_locs <- array(data = NA, dim = c(20, 3));
for(i in 1:20){
ind1_locs[i, 1] <- i # Save the time step
ind1_locs[i, 2] <- inds_hist[[i]][1, 2]; # xloc for the time step
ind1_locs[i, 3] <- inds_hist[[i]][1, 3]; # yloc for the time step
}
colnames(ind1_locs) <- c("time_step", "x_loc", "y_loc");
print(ind1_locs);
## time_step x_loc y_loc
## [1,] 1 4 2
## [2,] 2 4 1
## [3,] 3 5 1
## [4,] 4 6 2
## [5,] 5 5 2
## [6,] 6 5 1
## [7,] 7 4 2
## [8,] 8 4 1
## [9,] 9 4 2
## [10,] 10 3 3
## [11,] 11 3 4
## [12,] 12 3 5
## [13,] 13 2 6
## [14,] 14 2 6
## [15,] 15 3 6
## [16,] 16 2 7
## [17,] 17 2 8
## [18,] 18 2 7
## [19,] 19 2 7
## [20,] 20 1 6
The array above shows the full path of individual 1 from x location 4 and y location 2 to x location 1 and y location 6. We could do the same for any number of individuals in the model, if desired, and for any number of time steps. We therefore have a working model of individual movement within a confined area of landscape. In the next section, we will add some more biological realism by creating a new rule and function for modelling individual births in the population.
5. Simulating individual birth
To simulate birth in our population of individuals, we need some rule to determine when an individual reproduces. As a simplifying assumption, let as assume that all individuals in our model are female, and that the number of birth events for an individual Bi is sampled from a Poisson distribution with 0.5 expected offspring per time step (recall that a Poisson distribution describes the number of events occurring over a fixed interval of time),
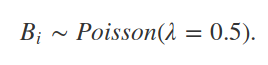
In R, we can sample some number of values from a Poisson distribution with a given rate parameter (λ, defining both the mean and variance of the Poisson distribution) using the rpois function. If we wanted to sample for five individuals with λ = 0.5, we could therefore use the code below.
rpois(n = 5, lambda = 0.5);
## [1] 0 0 0 0 0
If we are simulating individual reproduction, however, there needs to be some place in the model to store these values. The simplest solution is to initialise our array of individuals (inds) with another column, representing the number of offspring an individual has produced at a given time step.
inds <- array(data = 0, dim = c(5, 4));
colnames(inds) <- c("body_mass", "x_loc", "y_loc", "repr");
rownames(inds) <- c("ind_1", "ind_2", "ind_3", "ind_4", "ind_5");
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[,2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[,3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
## body_mass x_loc y_loc repr
## ind_1 20.76323 4 7 0
## ind_2 22.48684 6 8 0
## ind_3 22.14153 1 1 0
## ind_4 21.69553 3 2 0
## ind_5 21.13485 6 4 0
We now have the added column repr for individual reproduction. We could add some reproduction for each individual immediately by sampling from rpois to add values to the fourth column, but for now I will instead create a new function birth to both sample births for each individual and add the new offspring at the same time. In the birth function, each individual will first be assigned a number of offspring with rpois, then these new offspring will be added to the array inds.
birth <- function(inds, lambda = 0.5, repr_col = 4){
total_inds <- dim(inds)[1]; # Get the number of individuals in inds
ind_cols <- dim(inds)[2]; # Total inds columns
inds[, repr_col] <- rpois(n = total_inds, lambda = lambda);
total_off <- sum(inds[, repr_col]);
# ---- We now have the total number of new offspring; now add to inds
new_inds <- array(data = 0, dim = c(total_off, ind_cols));
new_inds[,1] <- rnorm(n = dim(new_inds)[1], mean = 23, sd = 3);
new_inds[,2] <- sample(x = 1:8, size = dim(new_inds)[1], replace = TRUE);
new_inds[,3] <- sample(x = 1:8, size = dim(new_inds)[1], replace = TRUE);
# ---- Our new offspring can now be attached in the inds array
inds <- rbind(inds, new_inds);
return(inds);
}
In the function above, we first get the dimensions of the ind array (total_inds and ind_cols). Next, we sample from a Poisson distribution using rpois to determine how many offspring each individual has produced. Next, we sum that number up to get total_off, which will be the total number of new individuals to be added to inds. A new array is made (new_inds) with total_off rows and ind_cols columns, and values are initialised for individual body mass and location. Finally, this new_inds array is combined with the old one inds using rbind[2] so that our working array of individuals now has both the old individuals and the newly born ones. We can see how this function runs now by running it below.
inds <- birth(inds = inds);
What has happend above is that we have read inds into the function birth and stored the output into an (overwritten) inds with the new individuals in the array.
## body_mass x_loc y_loc repr
## ind_1 20.76323 4 7 0
## ind_2 22.48684 6 8 0
## ind_3 22.14153 1 1 1
## ind_4 21.69553 3 2 1
## ind_5 21.13485 6 4 1
## 24.28716 8 2 0
## 23.87505 1 8 0
## 17.86060 1 7 0
Note that the sum of the fourth column equals the number of new individuals added (3). The new individuals do not have row names, but this is okay; we do not really need them. Note that there are other ways that we could add to births. We might, for example, wish for new offspring to have similar body masses to their mothers’, or for offspring to be initialised in the same locations as their mothers rather than being randomly placed on the landscape. I encourage the reader to try to think about these kinds of possibilities, and to try adding them to the code above.
For now, we can simulation both birth and movement of inds over time using the loop below (note that the code below is the same as that from the previous section, with some modifications). Instead of 20 time steps, I will only simulate 10 for now. I will also, arbitrarily, choose to model birth as occurring after movement in the population.
ts <- 0;
time_steps <- 10;
inds_hist <- NULL;
while(ts < time_steps){
inds <- movement(inds);
inds <- birth(inds);
ts <- ts + 1;
inds_hist[[ts]] <- inds;
}
The above has effectively simulated unrestricted population growth; individuals do not even die. Hence, even with a fairly low birth rate, by the end of the simulation there are 576 total individuals in the population. This is already too many individuals to print the entire array, but we can at least plot the abundance of individuals over time. Recall how we looped through the list inds_hist in the previous section to pull out the location of the first individual in each time step. We can do the same for pulling out abundance of individuals (number of rows in the array) for each time step.
ind_abund <- array(data = NA, dim = c(10, 2));
for(i in 1:10){
ind_abund[i, 1] <- i; # Save the time step
ind_abund[i, 2] <- dim(inds_hist[[i]])[1]; # rows in inds_hist[[i]]
}
colnames(ind_abund) <- c("time_step", "abundance");
print(ind_abund);
## time_step abundance
## [1,] 1 14
## [2,] 2 21
## [3,] 3 34
## [4,] 4 49
## [5,] 5 70
## [6,] 6 111
## [7,] 7 173
## [8,] 8 260
## [9,] 9 386
## [10,] 10 576
We can see from the table above that the population size is increasing exponentially. If we were to run too many time steps, we might risk simulating more individuals than the memory of our computer can store, ultimately causing a crash. This is obviously a problem computationally, but it is also biologically unrealistic. We need some sort of density regulation in the population. In the next section, we will apply a new rule to model individual death.
6. Simulating individual death
We can simulate individual death similarly to individual birth. Here though, because we want death to be density dependent, the probability of individual death needs to somehow be related to total population abundance. There are several reasonable ways to do model death in this way. For example, we could a write function that checks population abundance, assigns the probability of individual death based on some carrying capacity, then realises death by a Bernoulli trial (sampling a 0 or 1) using that assigned probability (i.e., as total abundance increases, each individual’s probability of dying also increases, and we sample each individual’s survival versus mortality from this probability to see if they make it into the next time step using something like rbinom). Another, simpler, option is to relate mortality to the number of individuals on a landscape cell.
Suppose we assume that each landscape cell has enough resources to feed one individual for one time step. We might then write a function death that causes mortality whenever there is more than one individual on the same landscape cell. Because there are 8 × 8 = 64 total landscape cells, the maximum possible population size of would then be 64. This would control the population size in a biologically intuitive way. To get started, I will reinitialise some individuals below, this time with another column added, which will represent mortality.
inds <- array(data = 0, dim = c(5, 5));
colnames(inds) <- c("body_mass", "x_loc", "y_loc", "repr", "death");
rownames(inds) <- c("ind_1", "ind_2", "ind_3", "ind_4", "ind_5");
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[,2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[,3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
## body_mass x_loc y_loc repr death
## ind_1 24.18466 8 1 0 0
## ind_2 20.61052 3 3 0 0
## ind_3 23.91818 3 7 0 0
## ind_4 26.34729 3 4 0 0
## ind_5 27.03324 3 1 0 0
Writing a function for mortality will be a bit more challenging that writing a function for birth. The code here will need to loop through each landscape cell, check to see how many individuals are on the cell, and if there is more than one individual on the cell, to select only one individual to survive.
death <- function(inds, xlen = 8, ylen = 8, dcol = 5, xcol = 2, ycol = 3){
for(xdim in 1:xlen){ # For each row `xdim` of the landscape...
for(ydim in 1:ylen){ # For each col `ydim` of the landscape...
# Get the total number of individuals on the landscape cell
on_cell <- sum( inds[, xcol] == xdim & inds[, ycol] == ydim);
# Only do something if on_cell is more than one
if(on_cell > 1){
# Get all of the occupants (by row number) on the cell
occupants <- which(inds[, xcol] == xdim & inds[, ycol] == ydim);
# Sample all but one random occupant to die
rand_occ <- sample(x = occupants, size = on_cell - 1);
# Then add their death to the last column of inds
inds[rand_occ, dcol] <- 1;
}
}
}
return(inds);
}
I have used a few tricks of R to keep the code short here, but I want to walk the reader through what is happening. As already mentioned, the double four loop cycles through each cell of the landscape. The outer loop goes through each x location from 1 to ylen (default value of 8). For each x location, the inner loop goes through each y location (so first x = 1 and y = 1, then x = 1 and y = 2, and so forth until we get to x = 2 and y = 1, then x = 2 and y = 2, etc.). The line returning on_cell gets the total number of individuals that have an x location of xdim and a y location of ydim. If this number is more than one, then mortality needs to happen. The line returning occupants finds the individuals on the cell of interest using the which function in R, then the sample function is used to sample all but one of these individuals (sampling without replacement). In each case, occupants and rand_occ are vectors of numbers identifying individuals, and these numbers correspond to the individuals’ rows. Hence individuals in the rows rand_occ will die, so we add a 1 to the death column.
To test this function, we can set individuals 2 and 3 to have the same location as individual 1. If the function is working, then only one of these three individuals should survive after running the function death.
inds[2, 2] <- inds[1, 2]; # Individiual 2 now in same x location as 1
inds[2, 3] <- inds[1, 3]; # Individiual 2 now in same x location as 1
inds[3, 2] <- inds[1, 2]; # Individiual 3 now in same x location as 1
inds[3, 3] <- inds[1, 3]; # Individiual 3 now in same x location as 1
## body_mass x_loc y_loc repr death
## ind_1 24.18466 8 1 0 0
## ind_2 20.61052 8 1 0 0
## ind_3 23.91818 8 1 0 0
## ind_4 26.34729 3 4 0 0
## ind_5 27.03324 3 1 0 0
We can now run death below, then print inds again.
inds <- death(inds = inds);
print(inds);
## body_mass x_loc y_loc repr death
## ind_1 24.18466 8 1 0 1
## ind_2 20.61052 8 1 0 1
## ind_3 23.91818 8 1 0 0
## ind_4 26.34729 3 4 0 0
## ind_5 27.03324 3 1 0 0
Note that two of the individuals above are dead (ones in column 5), and the rest have survived (zeros in column 5). We can retain the living individuals using the code below, which re-defines inds as only the subset of inds rows in which the value of column 5 is 0.
inds <- inds[inds[, 5] == 0,]; # Retains individuals that did not die
print(inds);
## body_mass x_loc y_loc repr death
## ind_3 23.91818 8 1 0 0
## ind_4 26.34729 3 4 0 0
## ind_5 27.03324 3 1 0 0
We can now include the death function to simulate a population with movement, birth, and death. The code below simulates a population over 20 time steps.
# ----- Initialise individuals
inds <- array(data = 0, dim = c(5, 5));
colnames(inds) <- c("body_mass", "x_loc", "y_loc", "repr", "death");
rownames(inds) <- c("ind_1", "ind_2", "ind_3", "ind_4", "ind_5");
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[,2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[,3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
# ---- Start the simulation as before
ts <- 0;
time_steps <- 20;
inds_hist <- NULL;
while(ts < time_steps){
inds <- movement(inds);
inds <- birth(inds);
inds <- death(inds);
inds <- inds[inds[, 5] == 0,]; # Retain living
ts <- ts + 1;
inds_hist[[ts]] <- inds;
}
This is now a working individual-based model. We have incorporated the biological processes of movement, births, and deaths to simulate a discrete population over time. We can use the same code as from the previous section to summarise how population abundance has changed over time.
ind_abund <- array(data = NA, dim = c(20, 2));
for(i in 1:20){
ind_abund[i, 1] <- i; # Save the time step
ind_abund[i, 2] <- dim(inds_hist[[i]])[1]; # rows in inds_hist[[i]]
}
colnames(ind_abund) <- c("time_step", "abundance");
print(ind_abund);
## time_step abundance
## [1,] 1 6
## [2,] 2 8
## [3,] 3 10
## [4,] 4 10
## [5,] 5 19
## [6,] 6 22
## [7,] 7 24
## [8,] 8 26
## [9,] 9 29
## [10,] 10 34
## [11,] 11 38
## [12,] 12 44
## [13,] 13 44
## [14,] 14 40
## [15,] 15 31
## [16,] 16 31
## [17,] 17 31
## [18,] 18 35
## [19,] 19 37
## [20,] 20 36
Compare the population abundances here with those from the previous section. By adding death to our model, and allowing only one individual to survive per cell, the size of the simulated population is no longer increasing exponentially. We can take a look at the individuals on the simulated landscape below.
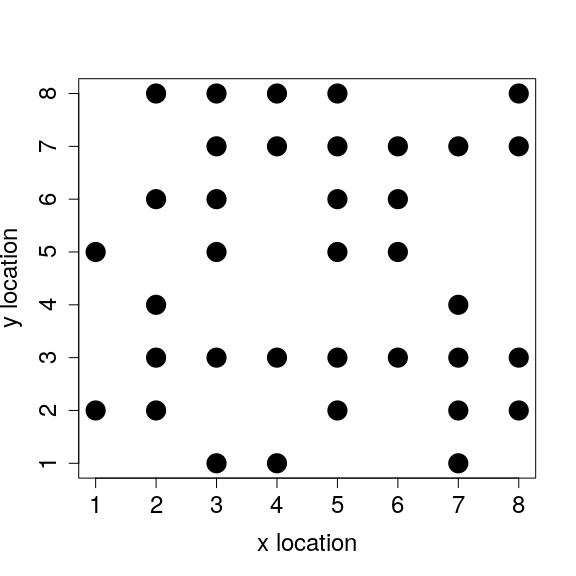
Notice that the entire landscape has not filled up. Since newly born individuals are placed at random on the landscape, and individuals also move randomly, it is unlikely that the landscape will become saturated with individuals. We have effectively simulated a process of intra-specific competition; that is, competition among individuals within the same species for a limiting resource (space, in this case, in the form of landscape cells). If we wanted to investigate intra-specific competition more closely, we could change some of the assumptions of our IBM. Such assumptions could include the size of the landscape, the number individuals that could survive on the same landscape cell, or perhaps how individuals move from cell to cell (e.g., to avoid competition). We could also add new biological processes using the current code as a starting point. For example, we might be interested in how body mass evolves as a consequence of intra-specific competition. To investigate this, we would need to make body_mass heritable in our model (i.e., make offspring body_mass more similar to parent body_mass than expected by chance, probably by adding some code in the birth function), and we would need to have some rule relating body_mass to the outcome of intra-specific competition (e.g., by making individuals with high body_mass more likely to survive than individuals with low body_mass, probably by adding some code in the death function). These are good exercises to try, and I encourage the reader to make modifications like this to practice coding IBMs.
Having a working population of individuals, there is one more exercise that I want to present. In the next section, I will demonstrate how we can use a second array to model a population of predators on inds. And by the end of the next section, we will have a working model of predator-prey dynamics on a spatially explicit landscape.
7. Predator-prey dynamics
In this section, I will show how to write a simple model of predation. Much of the work has actually already been done. We already have data structures for individuals (an array), and functions for movement, birth, and death. We can use these same structures and functions for modelling predators, writing new code only where there is a meaningful difference between predators and prey. We can start with a new array pred, which is of the same format as inds and contains five predators.
pred <- array(data = 0, dim = c(5, 5));
colnames(pred) <- c("body_mass", "x_loc", "y_loc", "repr", "death");
rownames(pred) <- c("pred_1", "pred_2", "pred_3", "pred_4", "pred_5");
pred[,1] <- rnorm(n = dim(pred)[1], mean = 23, sd = 3);
pred[,2] <- sample(x = 1:8, size = dim(pred)[1], replace = TRUE);
pred[,3] <- sample(x = 1:8, size = dim(pred)[1], replace = TRUE);
## body_mass x_loc y_loc repr death
## pred_1 27.90536 5 3 0 0
## pred_2 22.91469 7 4 0 0
## pred_3 29.35038 5 1 0 0
## pred_4 30.24029 8 4 0 0
## pred_5 24.48636 1 6 0 0
Note that because the pred array is of the same structure as the inds array, all of the functions that we wrote (movement, birth, and death) could equally well be used for our predators. But because we are modelling predation, this might not always make sense. We might, for example, be willing to assume that predators move similarly as their prey, but want to model birth and death processes differently. It would be reasonable to assume that birth and death are both affected by how successful a predator is at acquiring prey, so perhaps we only want predators to survive and breed if they have eaten some fixed number of times. For simplicity, I will assume that predators that eat at least one prey in inds survive to the next time step, while predators that eat two prey reproduce. I will further assume that predators cannot eat more than two prey in a time step, and that predators that fail to eat die. Note that given these assumptions, it is necessary to write new code to model predator birth and death, but before doing this, it is worth thinking about the order of operations in the code. That is, it will be useful to consider when predation will happen (e.g., at the start or end of a time step). Consider again the code from the previous section used to simulate individuals in the population.
# ----- Initialise individuals
inds <- array(data = 0, dim = c(5, 5));
colnames(inds) <- c("body_mass", "x_loc", "y_loc", "repr", "death");
rownames(inds) <- c("ind_1", "ind_2", "ind_3", "ind_4", "ind_5");
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[,2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[,3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
# ---- Start the simulation as before
ts <- 0;
time_steps <- 20;
inds_hist <- NULL;
while(ts < time_steps){
inds <- movement(inds);
inds <- birth(inds);
inds <- death(inds);
inds <- inds[inds[, 5] == 0,]; # Retain living
ts <- ts + 1;
inds_hist[[ts]] <- inds;
}
In the code above, individuals (now prey) move, give birth, then die. And given the rules of individual mortality, only one individual, at most, is allowed per landscape cell at the end of a time step. Hence, if predation were to happen at the end of the time step, predators could not reproduce. Biologically, having predation happen after prey death as caused by density dependence might also not make much sense. A good argument could probably be made for having predators move and eat first in the time step (i.e., preceding individual movement, birth, and death within the while loop above), both on biological and modelling grounds. I do not want to dwell on this, but only point out that thinking about the order in which events occur in an IBM is important, and therefore should be considered carefully. It is often good to change the order of events to see how it affects simulation results. For now, I will assume that predators get to move, eat, and reproduce after prey give birth but before prey die. It is only at this point, between birth and death above, in which more than two individuals can exist on the same cell, so this is the only time that predators could possibly eat more than one prey item to reproduce.
Predator movement is easy because we already have the movement function, and are content to assume that predators move in the same way as prey, potentially moving one landscape cell in any random direction.
pred <- movement(pred);
Birth and death requires a bit more work. We need a function that checks to see how many prey are located on a predator’s landscape cell. If that number is zero, then the predator should die. If that number is greater than zero, then the predator should survive. And if that number is greater than one, then the predator should reproduce. We also need to make sure that the eaten prey are removed from inds so that they do not reproduce, or are tagged in some way so that they can be removed in the death function.
predation <- function(pred, inds, xcol = 2, ycol = 3, rcol = 4, dcol = 5){
predators <- dim(pred)[1]; # Predator number
pred[, dcol] <- 1; # Assume dead until proven otherwise
pred[, rcol] <- 0; # Assume not reproducing until proven otherwise
for(p in 1:predators){ # For each predator (p) in the array
xloc <- pred[p, xcol]; # Get the x and y locations
yloc <- pred[p, ycol];
N_prey <- sum( inds[, xcol] == xloc & inds[, ycol] == yloc);
# ----- Let's take care of the predator first below
if(N_prey > 0){
pred[p, dcol] <- 0; # The predator lives
}
if(N_prey > 1){
pred[p, rcol] <- 1; # The predator reproduces
}
# ----- Now let's take care of the prey
if(N_prey > 0){ # If there are some prey, find them
prey <- which( inds[, xcol] == xloc & inds[, ycol] == yloc);
if(N_prey > 2){ # But if there are more than 2, just eat 2
prey <- sample(x = prey, size = 2, replace = FALSE);
}
inds[prey, dcol] <- 1; # Record the prey as dead
}
} # We now know which inds died, and which prey died & reproduced
# ---- Below removes predators that have died
pred <- pred[pred[,dcol] == 0,] # Only survivors now left
# ----- Below adds new predators based on the reproduction above
pred_off <- sum(pred[, rcol]);
new_pred <- array(data = 0, dim = c(pred_off, dim(pred)[2]));
new_pred[,1] <- rnorm(n = dim(new_pred)[1], mean = 23, sd = 3);
new_pred[,2] <- sample(x = 1:8, size = dim(new_pred)[1], replace = TRUE);
new_pred[,3] <- sample(x = 1:8, size = dim(new_pred)[1], replace = TRUE);
pred <- rbind(pred, new_pred);
# ----- Now let's remove the prey that were eaten
inds <- inds[inds[,dcol] == 0,]; # Only living prey left
# Now need to return *both* the predator and prey arrays
pred_prey <- list(pred = pred, inds = inds);
return(pred_prey);
}
That is a lot of code, but at the end of all of it, we should have what we want. Predator and prey arrays go into the function. Predators that do not feed and therefore die are removed from their array (pred), while predators that feed and reproduce add a new offspring to the array. Prey are removed from their array (inds) if they are eaten. Note that, for the first time, we need to return more than one array. Both the inds and the pred array are changed in the function above, so a list is returned with both arrays as list elements. To access each of these elements, we can use pred_prey$pred and pred_prey$inds for predators and prey, respectively.
To test the function written above, we can put some individuals and predators down in a known location, then check to see that predation has happened as intended. I will initialise a new inds with six total individuals: three individuals at x = 1, y = 1, two individuals at x = 2, y = 1, and one individual at x = 3, y = 1.
inds <- array(data = 0, dim = c(6, 5));
colnames(inds) <- c("body_mass", "x_loc", "y_loc", "repr", "death");
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[1:3, 2] <- 1; # x location
inds[1:3, 3] <- 1; # y location
inds[4:5, 2] <- 2;
inds[4:5, 3] <- 1;
inds[6, 2] <- 3;
inds[6, 3] <- 1;
## body_mass x_loc y_loc repr death
## [1,] 20.06693 1 1 0 0
## [2,] 19.69997 1 1 0 0
## [3,] 16.50927 1 1 0 0
## [4,] 22.34860 2 1 0 0
## [5,] 26.93147 2 1 0 0
## [6,] 25.51669 3 1 0 0
Now, I will add a single predator to each of four locations.
pred <- array(data = 0, dim = c(4, 5));
colnames(pred) <- c("body_mass", "x_loc", "y_loc", "repr", "death");
pred[,1] <- rnorm(n = dim(pred)[1], mean = 23, sd = 3);
pred[1, 2] <- 1; # x location
pred[1, 3] <- 1; # y location
pred[2, 2] <- 2;
pred[2, 3] <- 1;
pred[3, 2] <- 3;
pred[3, 3] <- 1;
pred[4, 2] <- 4;
pred[4, 3] <- 1;
## body_mass x_loc y_loc repr death
## [1,] 23.87095 1 1 0 0
## [2,] 14.50862 2 1 0 0
## [3,] 22.20024 3 1 0 0
## [4,] 24.83630 4 1 0 0
We now see that there are four occupied landscape cells, each with a single predator. Based on the rules that we specified in the function predation above, we can predict what will happen on each of these cells as a consequence of the local abundance of prey. The code below runs the function, then stores the new arrays back into inds and prey.
pred_prey <- predation(inds = inds, pred = pred);
inds <- pred_prey$inds;
pred <- pred_prey$pred;
Here is what the individual array now looks like.
## body_mass x_loc y_loc repr death
## 16.50927 1.00000 1.00000 0.00000 0.00000
There is only one surviving prey. This is one of the prey on the landscape cell x = 1, y = 1, which started out with three individuals on which two other individuals were eaten by the predator. All other individuals were eaten because they occupied cells with two or fewer other individuals. Below is what the predator array now looks like.
## body_mass x_loc y_loc repr death
## [1,] 23.87095 1 1 1 0
## [2,] 14.50862 2 1 1 0
## [3,] 22.20024 3 1 0 0
## [4,] 22.42758 3 3 0 0
## [5,] 21.47514 4 8 0 0
Note that two predators have reproduced; each of these predators was on a cell with two or more individuals that became prey. The predator on the cell with only one individual (x = 3, y = 1) survived but did not reproduce, while the predator on the cell with no individuals did not survive (x = 4, y = 1). Note that there are also newly born predators in pred above (rows 4 and 5), as was expected.
If we are confident that the predation function is working as intended, we can now include it into the simulation to model the dynamics of predators and prey over time.
8. Putting it all together
Having coded functions for movement, birth, death, and predation, we can now put all of these processes together to simulate a predator-prey system. The code for simulating these processes will build off of some of the code above, as I introduced originally to simulate individual movement over time and most recently used when considering the order of predation in the previous section. All that we need to add is the initialisation of predators, the inclusion of predator movement and predation in each time step, and the recording of the predator array over time steps.
In the code below, 40 individuals are initialised; the high number ensures that there are enough prey to sustain the predator population. The predator abundance is initialised at five, and both prey and predators are randomly placed on the landscape. Within the while loop below, we now include predator movement (movement(pred)) and predation (predation(pred = pred, inds = inds)). The list pred_hist now stores the pred array in each time step, as is done for the inds array, so that we can reconstruct predator movement and abundances over time. Hence, by running the code below, we can analyses both inds_hist and pred_hist to understand predator-prey dynamics (note that the below code will sometimes lead to predator or prey extinction, causing an error in R; we do not have a rule for what happens if extinctions occurs, but it would be useful to think of one to avoid having to deal with error messages).
# ----- Initialise individuals (prey)
inds <- array(data = 0, dim = c(40, 5));
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[,2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[,3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
# ----- Initialise individuals (predator)
pred <- array(data = 0, dim = c(20, 5));
pred[,1] <- rnorm(n = dim(pred)[1], mean = 23, sd = 3);
pred[,2] <- sample(x = 1:8, size = dim(pred)[1], replace = TRUE);
pred[,3] <- sample(x = 1:8, size = dim(pred)[1], replace = TRUE);
# ---- Start the simulation as before
ts <- 0;
time_steps <- 40;
inds_hist <- NULL;
pred_hist <- NULL;
while(ts < time_steps){
pred <- movement(pred);
inds <- movement(inds); # Note: I increased prey birth rate
inds <- birth(inds, lambda = 1.5);
pred_prey_res <- predation(pred = pred, inds = inds);
pred <- pred_prey_res$pred;
inds <- pred_prey_res$inds;
inds <- death(inds);
inds <- inds[inds[, 5] == 0,]; # Retain living
ts <- ts + 1;
inds_hist[[ts]] <- inds;
pred_hist[[ts]] <- pred;
}
We could look at any time step in inds_hist and pred_hist. For example, if we wanted to see predators in the tenth time step, then we could type pred_hist[[10]] in the R console. Instead, suppose what we are really interested in is how predator and prey population abundances change over time. We can loop through inds_hist and pred_hist in the same way that we did with just inds_hist before to gather population abundance prior to including predators. Now, however, we need to include a column in the summary array (ind_abund) that includes predator abundance. The code below will generate a new array with three columns. Column 1 will show the time step, column 2 will show prey abundance, and column 3 will show predator abundance.
ind_abund <- array(data = NA, dim = c(40, 3));
for(i in 1:40){
ind_abund[i, 1] <- i; # Save the time step
ind_abund[i, 2] <- dim(inds_hist[[i]])[1]; # rows in inds_hist[[i]]
ind_abund[i, 3] <- dim(pred_hist[[i]])[1]; # rows in pred_hist[[i]]
}
colnames(ind_abund) <- c("time_step", "abundance", "predators");
print(ind_abund);
## time_step abundance predators
## [1,] 1 41 23
## [2,] 2 39 35
## [3,] 3 32 45
## [4,] 4 28 50
## [5,] 5 20 50
## [6,] 6 17 34
## [7,] 7 19 20
## [8,] 8 24 15
## [9,] 9 28 13
## [10,] 10 40 17
## [11,] 11 40 22
## [12,] 12 40 25
## [13,] 13 42 33
## [14,] 14 35 48
## [15,] 15 27 52
## [16,] 16 26 56
## [17,] 17 24 42
## [18,] 18 27 35
## [19,] 19 25 32
## [20,] 20 25 26
## [21,] 21 25 20
## [22,] 22 30 21
## [23,] 23 37 18
## [24,] 24 43 27
## [25,] 25 40 34
## [26,] 26 39 49
## [27,] 27 27 58
## [28,] 28 13 57
## [29,] 29 9 25
## [30,] 30 14 8
## [31,] 31 20 4
## [32,] 32 26 4
## [33,] 33 41 4
## [34,] 34 51 6
## [35,] 35 56 8
## [36,] 36 53 9
## [37,] 37 48 11
## [38,] 38 53 17
## [39,] 39 45 27
## [40,] 40 38 42
Note that there is an interesting pattern in the predator and prey dynamics. It seems that both the predator and prey oscillate from low abundance to high abundance. The plot below shows this oscillation more clearly
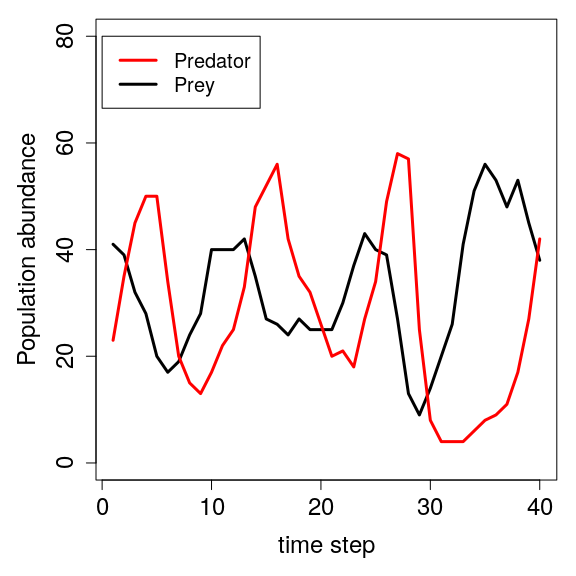
In the plot above, we see that predator and prey population abundances oscillate over time. As prey become more abundant, predator growth rate increases. As predators then become more common, prey abundance in turn starts to fall. Note that these oscillations are the same kinds of limit cycles predicted by mathematical models using Lotka-Volterra equations describing the change in prey (x) and predator (y) densities over time (t),
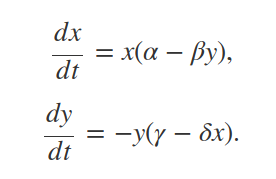
But we have not used either of these equations when building our individual-based model! We have not even set parameters such as α, β, δ, or γ in the code. So what is going on here? The answer is that both models make similar biological assumptions – similar enough to produce similar oscillating patterns of predator-prey abundances (at least, for a subset of parameter values). For example, in both models, the growth rate of one population is tied to the density of the other population. Using different modelling techniques is often a useful way to address theoretical questions from slightly different perspectives using different biological assumptions (Caswell 1988).
9. Conclusions
Individual-based models are highly flexible tools for modelling biological systems. These notes are only a starting point for developing and using them, and I hope that they provide a helpful foundation for anyone interested in coding their own IBMs. I have tried to make the code as readable as possible for newcomers to IBMs and coding in R, particularly in the first few sections. Consequently, some of the code is not as efficient as it could be in terms of computational efficiency. Nevertheless, I encourage interested readers to experiment with the code (aggregated below), and to add new lines, functions, or data structures to try to model different biological processes. Some potentially interesing starting points might include the below.
- Modelling a torus landscape within
movement, rather than a landscape with a reflecting boundary (i.e., make individuals that leave on one side of the landscape come back on the other side). - Modelling the heritability of body mass by causing individuals to give birth to offspring have body masses similar to their parents.
- Modelling disturbance on the landscape to see how high mortality on a subset of landscape cells affects global predator-prey dynamics (this would likely require a new function).
- Modelling birth or death rate as dependent upon the quality of a landscape cell (this would likely require a new data structure to store landscape cell quality).
The ability to model biological processes such as the ones above can be very useful, and a lot of fun. IBMs can also be extremely helpful for clarifying how we think about biological systems, both to develop general theory and to predict the dynamics of specific complex systems (e.g., Uchmański and Grimm 1996; McLane et al. 2011; Benton 2012; Aben et al. 2014; Duthie et al. 2018; Bocedi and Reid 2017).
10. Appendix with all code
It is useful to have all of the code in one place. Below, I have included all of the functions and code required to run the above predator-prey individual-based model. The code below can be highlighted, copied, and run in the R console to produce a simulation over 40 time steps.
# =============================================================================
# Movement function
# =============================================================================
movement <- function(inds, xloc = 2, yloc = 3, xmax = 8, ymax = 8){
total_inds <- dim(inds)[1]; # Get the number of individuals in inds
move_dists <- c(-1, 0, 1); # Define the possible distances to move
x_move <- sample(x = move_dists, size = total_inds, replace = TRUE);
y_move <- sample(x = move_dists, size = total_inds, replace = TRUE);
inds[, xloc] <- inds[, xloc] + x_move;
inds[, yloc] <- inds[, yloc] + y_move;
# ========= The reflecting boundary is added below
for(i in 1:total_inds){ # For each individual i in the array
if(inds[i, xloc] > xmax){ # If it moved passed the maximum xloc
inds[i, xloc] <- xmax - 1; # Then move it back toward the centre
}
if(inds[i, xloc] < 1){ # If it moved below 1 on xloc
inds[i, xloc] <- 2; # Move it toward the centre (2)
}
if(inds[i, yloc] > ymax){ # If it moved passed the maximum yloc
inds[i, yloc] <- ymax - 1; # Then move it back toward the centre
}
if(inds[i, yloc] < 1){ # If it moved below 1 on yloc
inds[i, yloc] <- 2; # Then move it toward the centre (2)
}
}
# ========= Now all individuals should stay on the landscape
return(inds);
}
# =============================================================================
# Birth function
# =============================================================================
birth <- function(inds, lambda = 0.5, repr_col = 4){
total_inds <- dim(inds)[1]; # Get the number of individuals in inds
ind_cols <- dim(inds)[2]; # Total inds columns
inds[, repr_col] <- rpois(n = total_inds, lambda = lambda);
total_off <- sum(inds[, repr_col]);
# ---- We now have the total number of new offspring; now add to inds
new_inds <- array(data = 0, dim = c(total_off, ind_cols));
new_inds[,1] <- rnorm(n = dim(new_inds)[1], mean = 23, sd = 3);
new_inds[,2] <- sample(x = 1:8, size = dim(new_inds)[1], replace = TRUE);
new_inds[,3] <- sample(x = 1:8, size = dim(new_inds)[1], replace = TRUE);
# ---- Our new offspring can now be attached in the inds array
inds <- rbind(inds, new_inds);
return(inds);
}
# =============================================================================
# Death function
# =============================================================================
death <- function(inds, xlen = 8, ylen = 8, dcol = 5, xcol = 2, ycol = 3){
for(xdim in 1:xlen){ # For each row `xdim` of the landscape...
for(ydim in 1:ylen){ # For each col `ydim` of the landscape...
# Get the total number of individuals on the landscape cell
on_cell <- sum( inds[, xcol] == xdim & inds[, ycol] == ydim);
# Only do something if on_cell is more than one
if(on_cell > 1){
# Get all of the occupants on the cell
occupants <- which(inds[, xcol] == xdim & inds[, ycol] == ydim);
# Sample all but one random occupants to die
rand_occ <- sample(x = occupants, size = on_cell - 1);
# Then add their death to the last column of inds
inds[rand_occ, dcol] <- 1;
}
}
}
return(inds);
}
# =============================================================================
# Predation function
# =============================================================================
predation <- function(pred, inds, xcol = 2, ycol = 3, rcol = 4, dcol = 5){
predators <- dim(pred)[1]; # Predator number
pred[, dcol] <- 1; # Assume dead until proven otherwise
pred[, rcol] <- 0; # Assume not reproducing until proven otherwise
for(p in 1:predators){ # For each predator (p) in the array
xloc <- pred[p, xcol]; # Get the x and y locations
yloc <- pred[p, ycol];
N_prey <- sum( inds[, xcol] == xloc & inds[, ycol] == yloc);
# ----- Let's take care of the predator first below
if(N_prey > 0){
pred[p, dcol] <- 0; # The predator lives
}
if(N_prey > 1){
pred[p, rcol] <- 1; # The predator reproduces
}
# ----- Now let's take care of the prey
if(N_prey > 0){ # If there are some prey, find them
prey <- which( inds[, xcol] == xloc & inds[, ycol] == yloc);
if(N_prey > 2){ # But if there are more than 2, just eat 2
prey <- sample(x = prey, size = 2, replace = FALSE);
}
inds[prey, dcol] <- 1; # Record the prey as dead
}
} # We now know which inds died, and which prey died & reproduced
# ---- Below removes predators that have died
pred <- pred[pred[,dcol] == 0,] # Only survivors now left
# ----- Below adds new predators based on the reproduction above
pred_off <- sum(pred[, rcol]);
new_pred <- array(data = 0, dim = c(pred_off, dim(pred)[2]));
new_pred[,1] <- rnorm(n = dim(new_pred)[1], mean = 23, sd = 3);
new_pred[,2] <- sample(x = 1:8, size = dim(new_pred)[1], replace = TRUE);
new_pred[,3] <- sample(x = 1:8, size = dim(new_pred)[1], replace = TRUE);
pred <- rbind(pred, new_pred);
# ----- Now let's remove the prey that were eaten
inds <- inds[inds[,dcol] == 0,]; # Only living prey left
# Now need to return *both* the predator and prey arrays
pred_prey <- list(pred = pred, inds = inds);
return(pred_prey);
}
# =============================================================================
# Simulate predator-prey dynamics
# =============================================================================
# ----- Initialise individuals (prey)
inds <- array(data = 0, dim = c(40, 5));
inds[,1] <- rnorm(n = dim(inds)[1], mean = 23, sd = 3);
inds[,2] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
inds[,3] <- sample(x = 1:8, size = dim(inds)[1], replace = TRUE);
# ----- Initialise individuals (predator)
pred <- array(data = 0, dim = c(20, 5));
pred[,1] <- rnorm(n = dim(pred)[1], mean = 23, sd = 3);
pred[,2] <- sample(x = 1:8, size = dim(pred)[1], replace = TRUE);
pred[,3] <- sample(x = 1:8, size = dim(pred)[1], replace = TRUE);
# ---- Start the simulation as before
ts <- 0;
time_steps <- 40;
inds_hist <- NULL;
pred_hist <- NULL;
while(ts < time_steps){
pred <- movement(pred);
inds <- movement(inds); # Note: I increased prey birth rate
inds <- birth(inds, lambda = 1.5);
pred_prey_res <- predation(pred = pred, inds = inds);
pred <- pred_prey_res$pred;
inds <- pred_prey_res$inds;
inds <- death(inds);
inds <- inds[inds[, 5] == 0,]; # Retain living
ts <- ts + 1;
inds_hist[[ts]] <- inds;
pred_hist[[ts]] <- pred;
}
# =============================================================================
# Print the results
# =============================================================================
ind_abund <- array(data = NA, dim = c(40, 3));
for(i in 1:40){
ind_abund[i, 1] <- i; # Save the time step
ind_abund[i, 2] <- dim(inds_hist[[i]])[1]; # rows in inds_hist[[i]]
ind_abund[i, 3] <- dim(pred_hist[[i]])[1]; # rows in pred_hist[[i]]
}
colnames(ind_abund) <- c("time_step", "abundance", "predators");
print(ind_abund);
# =============================================================================
# Plot the results
# =============================================================================
par(mar = c(5, 5, 1, 1));
plot(x = ind_abund[,2], type = "l", lwd = 3, ylim = c(0, 80),
xlab = "time step", ylab = "Population abundance", cex.axis = 1.5,
cex.lab = 1.5);
points(x = ind_abund[,3], type = "l", lwd = 3, col = "red");
legend(x = 0, y = 80, legend = c("Predator", "Prey"), col = c("red", "black"),
cex = 1.25, lty = c("solid", "solid"), lwd = c(3, 3));
References
Aben, Job, Diederik Strubbe, Frank Adriaensen, Stephen C F Palmer, Justin M J Travis, Luc Lens, and Erik Matthysen. 2014. “Simple individual-based models effectively represent Afrotropical forest bird movement in complex landscapes.” Journal of Applied Ecology 51 (3): 693–702. doi:10.1111/1365-2664.12224.
Benton, T G. 2012. “Individual variation and population dynamics: lessons from a simple system.” Philosophical Transactions of the Royal Society London B 367 (1586): 200–210. doi:10.1098/rstb.2011.0168.
Bocedi, Greta, and Jane M Reid. 2017. “Feed-backs among inbreeding, inbreeding depression in sperm traits and sperm competition can drive evolution of costly polyandry.” Evolution. doi:10.1111/evo.13363.
Caswell, Hal. 1988. “Theory and models in ecology: a different perspective.” Ecological Modelling 43: 33–44.
DeAngelis, Donald L, and Wolf M Mooij. 2005. “Individual-based modeling of ecological and evolutionary processes.” Annual Review of Ecology, Evolution, and Systematics 36 (2005): 147–68. http://www.jstor.org/stable/10.2307/30033800.
Duthie, A Bradley, and Matthew R Falcy. 2013. “The influence of habitat autocorrelation on plants and their seed-eating pollinators.” Ecological Modelling 251: 260–70. doi:10.1016/j.ecolmodel.2012.12.019.
Duthie, A Bradley, Greta Bocedi, Ryan R Germain, and Jane M Reid. 2018. “Evolution of pre-copulatory and post-copulatory strategies of inbreeding avoidance and associated polyandry.” Journal of Evolutionary Biology. doi:10.1111/jeb.13189.
Grimm, Volker. 1999. “Ten years of individual-based modelling in ecology: what have we learned and what could we learn in the future?” Ecological Modelling 115 (2-3): 129–48. doi:10.1016/S0304-3800(98)00188-4.
McLane, Adam J., Christina Semeniuk, Gregory J. McDermid, and Danielle J. Marceau. 2011. “The role of agent-based models in wildlife ecology and management.” Ecological Modelling 222 (8). Elsevier B.V.: 1544–56. doi:10.1016/j.ecolmodel.2011.01.020.
Pettorelli, Nathalie, Jean Michel Gaillard, Guy Van Laere, Patrick Duncan, Petter Kjellander, Olof Liberg, Daniel Delorme, and Daniel Maillard. 2002. “Variations in adult body mass in roe deer: The effects of population density at birth and of habitat quality.” Proceedings of the Royal Society B 269 (1492): 747–53. doi:10.1098/rspb.2001.1791.
Uchmański, J, and Volker Grimm. 1996. “Individual-based modelling in ecology: what makes the difference?” Trends in Ecology & Evolution 11 (10): 437–41. http://www.sciencedirect.com/science/article/pii/0169534796200916.
[1]: This has happened to me once, on accident, when coding an IBM for plant-pollinator-exploiter interactions (Duthie and Falcy 2013). While testing my code, I had been printing off the locations of all individuals in the IBM to a plain text file. Later, upon running a large number of simulations, I noticed that my computer was slowing down quite a bit. Soon, a warning message came up that there was no more space to write to my hard drive. Because I had forgotten to stop printing all individual locations to the text file, every new location of millions of individuals was being recorded. The text file was well over 150 gigabytes when I forced the simulations to terminate, then removed the bit of code that printed individual locations.
[2]: For large IBMs, I feel obliged to point out that rbind can be very slow due to the way that memory is managed in R. If simulations start slowing down too much, it is best to refactor the code to avoid using rbind (or cbind). I only use it in the example here because it is a very convenient single line solution for binding two rows of an array together, and I do not want to get overwhelmed in the details of coding techniques for maximising efficiency.
